Introduction Of Protein
- Carbon, hydrogen, oxygen, and nitrogen make up the huge, complex organic molecules or compounds known as proteins.
- Proteins can be distinguished from carbohydrates and lipids by the presence of nitrogen. Some proteins also contain other elements besides nitrogen, such as sulfur, phosphorus, copper, and iron.
- A chain of amino acids is the building block of proteins.
- Proteins are type of macro-nutrients.
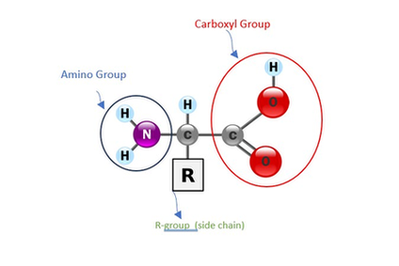
Basic Structure Of Protein
Proteins are those macro or biomolecules that serve as the structural units of our bodies and are composed of long chains of amino acids joined together by peptide bonds. For the formation of a protein, amino acids from any of the 21 distinct types can be combined. Peptide bond refers to the connection between the two amino acids. And, the functional group that distinguishes one amino acid from another is called the R-group.
- One amino acid has
- 1 carboxyl group acid (COOH)
- 1 amino group or nitrogen group (NH2)
- 1 hydrogen (H)
- and, 1 functional group (R)
Properties of Protein (Effect of Protein on Heat)
- Denaturation:
Proteins’ structural changes happen when they are subjected to heat, light, and/or pH changes. Denaturation is the term for these modifications to protein structure. It causes proteins’ solubility to alter. If the conditions that lead to it are moderate, it might be reversible, but most changes are irreversible. Daily occurrences of denaturing include boiling or heating meat and eggs, as well as milk curdling. - Coagulation:
When proteins are exposed to heat or a pH change, they coagulate. Heat is used to first denature the proteins before coagulating them.
Coagulation typically takes place within a temperature range of 65 to 90 degrees Celsius. Coagulation, in this context, refers to the transformation of a liquid protein solution into a solid or semi-solid mass. This process is characterized by curdling, bulk formation, congealing, or solidifying of the proteins involved.
For example, when raw egg, which is initially in a liquid state, is subjected to heat through methods like boiling or scrambling, the egg proteins coagulate. This coagulation results in the transformation of the fluid egg into a solid mass with a different texture and structure.
In another instance, when milk is incubated with curd, the lactic acid produced during the fermentation process coagulates the milk protein. This coagulation transforms the liquid milk into curd, a semi-solid product with a distinct texture and flavor. - Hydration of protein:
The effect of heat on proteins is closely tied to the process of protein hydration. Protein hydration refers to the binding of water molecules to the surface of proteins. In the realm of food science, this connection is crucially important.
Proteins have a number of functional groups with unshared electrons that can bind and attract the hydrogen atoms in water molecules. As a result, these protein groups start to bind with water molecules. As a result, these protein groups start to bind with water molecules. As a result of this initial bonding, aggregates of water molecules establish around each polar group on the protein molecule as a result of a cascade effect in which one bound water molecule attracts another bound water molecules.
The concentration of the protein, the pH of the solution, the temperature, and the presence of other compounds that may interact with water all have an impact on how much the protein is hydrated.
Gelatin gives a good example of how proteins are hydrated. Cold water causes gelatin to swell as a result of hydration. However, it breaks down into the water when heated. It solidifies once more when it cools. This characteristic enables the network of raised gelatin particles to retain water. Due to its capacity to create gels, serve as a whipping agent in the production of foam, and act as a clarifying agent in fruit juices—all made possible by its special hydration properties—gelatin is used in numerous food applications. - Gelation:
Gelation is the ability of proteins to form gels when they undergo a specific temperature treatment, typically heating followed by cooling. This process is essential in foods like jellies, puddings, and yogurts. When proteins denature and bond together, they create a 3D network that traps water and other ingredients, resulting in a firm and cohesive structure in these products.
When proteins are heated, they go through a process called denaturation, which causes them to separate from their native structure. This process is how gelation happens. These denatured proteins then have an a tendency to interact with one another, resulting in the formation of a three-dimensional network or matrix. This network not only interacts with one another but also draws the proteins closer together. As the network grows, it successfully captures water molecules and other elements within of its framework.
The outcome is a gel with a solid and consistent structure. With the stability, thickness, and form that consumers need, this gelation process gives a variety of foods the desired texture and consistency. - Emulsification:
Emulsification is a vital property of proteins when subjected to heat. This ability allows proteins to stabilize oil-water mixtures, which is crucial in various food products like mayonnaise, salad dressings, and ice cream.
During emulsification, proteins create a protective layer around oil droplets, effectively preventing them from coalescing and separating from the surrounding water. This protective layer ensures the mixture remains stable and maintains a smooth and consistent texture. This process plays a key role in the quality and texture of many beloved food items. - Foaming: This unique ability allows proteins to entrap air, giving rise to stable foams that adorn a variety of delectable dishes such as meringues, soufflés, and cakes. Under the influence of heat, proteins gracefully unfold, weaving an intricate network that captures and cradles air bubbles, creating a delicate, airy matrix.
It results light and airy desserts that delight both the palate and the eye, making foaming a crucial process in culinary creativity. - Viscosity :
Viscosity is the capacity of protein to thicken and give food items body. In many food products, including sauces, gravies, and soups, this is crucial. When proteins connect with one another and unfold, a network is formed that traps water and other components. Its texture becomes smooth and velvety as this network thickens.
Essential and Non-Essential Amino Acids
Based on nutritional requirements amino acids are grouped in two classes:
I. Essential Amino Acids
II. Non – Essential Amino Acids
- Essential Amino Acids
Those amino acids which can’t be synthesized in sufficient amounts by the body and must be provided by the diet are called essential amino acids. Out of 21 amino acids, there are 10 essential amino acids. Among those 9 are for adults with it 1 for children.- Histidine
- Methionine
- Threonine
- Tryptophan
- valine
- Phenylalanine
- Isoleucine
- Leucine
- Lysine
- Arginine (Additional for child)
Functions of Essential Amino Acids:
Histidine: This amino acid is necessary for the development of histamine, a neurotransmitter involved in a number of physiological activities, as well as for the growth and repair of tissues.
Isoleucine: Isoleucine is essential for the formation of hemoglobin, immune system activity, and muscle metabolism. Additionally, it helps keep blood sugar levels stable.
Leucine: Leucine is an essential component of muscle protein synthesis and plays a crucial role in both muscle development and recovery.
Lysine: Lysine is necessary for the development of collagen, the assimilation of calcium, and the synthesis of hormones, enzymes, and antibodies. Additionally, it aids in tissue healing.
Methionine: Methionine is an essential amino acid for metabolism and detoxification activities and a precursor to other significant compounds like cysteine and glutathione.
Phenylalanine: Phenylalanine is required for the synthesis of a number of neurotransmitters, such as norepinephrine, epinephrine, and dopamine. Furthermore, it functions as a precursor to the amino acid tyrosine.
Threonine: Threonine is important for preserving a healthy protein balance in the body and aids in the production of collagen and elastin, both of which are necessary for the health of the skin and connective tissues.
Tryptophan: A neurotransmitter that controls mood and sleep, serotonin, is made from tryptophan. Niacin (vitamin B3) synthesis depends on it as well.
Valine: Valine is particularly vital for stamina and muscle strength. It is also necessary for tissue healing and muscle coordination.
- Non-Essential Amino Acids
These amino acids can be synthesized in our body from other amino acids, so they are not dependent on food sources to be obtained. Altogether, there are 11 non-essential amino acids.
Namely: Cysteine, Serine, Tyrosine, Glutamic acid, Glutamine, Glycine, Proline, Alanine, Arginine, Asparagine, Aspartic acid.
Note: Non-essential amino acids are primarily produce in human liver.
Functions of Non-Essential Amino Acids:
Alanine: Alanine is necessary for the metabolism of glucose and provides energy during prolonged activity.
Asparagine: Asparagine is involved in the production of other amino acids and is necessary for preserving the body’s nitrogen balance.
Aspartic Acid: Aspartic acid plays a crucial role in the urea cycle, which gets rid of ammonia from the body.
Glutamic Acid: Glutamic acid is a neurotransmitter in the central nervous system that is important for cognitive function.
Serine: Serine participates in the metabolism of fats and fatty acids and serves as a precursor for the production of other amino acids.
Glycine: Glycine contributes to the synthesis of creatine, which is necessary for proper neuron and muscle function.
Proline: Proline is a part of collagen, which is essential for the health of the skin, joints, and connective tissues.
Cysteine: Cysteine is essential for the production of proteins, enzymes, and the antioxidant glutathione.
Tyrosine: Tyrosine serves as an important building block for the production of neurotransmitters like dopamine, adrenaline, and norepinephrine.
Arginine: Arginine participates in a number of metabolic processes, including the urea cycle and nitric oxide synthesis, which controls blood vessel function.
Glutamine: Glutamine is a main source of energy for the cells lining the digestive track and is necessary for the immune system.
While the body may produce non-essential amino acids, they can also be acquired through diet. But these amino acids are typically present in sufficient amounts in a balanced diet. Additionally, when the body’s need for some non-essential amino acids, such as glutamine, surpasses its capacity to produce them because of sickness or stress, they might turn conditionally essential.
| S. N | Characteristics | Essential Amino Acids | Non-Essential Amino Acids |
| 1. | Definition | It can’t be synthesized by human body. | It is synthesizable by the human body. |
| 2. | Requirement | It must be obtained from the daily diet. | It is not strictly required. |
| 3. | Other names | It is also known as indispensable amino acids. | It is also known as dispensable amino acids. |
| 4. | Synthesize | Adults can’t synthesize 9 amino acids. | Adult can synthetize 11 amino acids. |
| 5. | Variety | There are total 10 essential amino acids. | There are total 11 no-essential amino acids. |
| 6. | Examples | Example: Tryptophan, Lysine, Arginine, Leucine, etc. | Example: Proline, Glycine, Cysteine, Alanine, etc. |
| 7 | Health Impact | Deficiency can cause health issues and needs to be treated with eating habits. | Although deficiencies are less common, they can emerge under certain circumstances, such as illness or stress, necessitating the use of supplements. |
| 8 | Conditionally Essential | No, they are always essential | Can become conditionally essential under specific circumstances |
Classification of protein
On the basis of nutritive value of proteins,it can be classified as:-
- Complete Protein
- Incomplete Protein
- Partially Complete Protein
- Complete protein (High Quality Protein)
All of the necessary essential amino acids are present in these proteins in appropriate amounts and ratios to meet the body’s requirements. Even when served as the only protein supply, they continue to support life. They are regarded as high-quality proteins due to their nutritional completeness.
For example: milk, egg, meat, poultry products, fish and dairy products (like milk, cheese & yogurt) are all examples of proteins that are derived from animals. - Incomplete Protein
These proteins cannot sustain life on their own because they lack one or more essential amino acids.
Vegetables, fruits, beans, legumes, tofu, grains, pulses, nuts, and oilseeds are all plant sources of protein, and they all contain varied amounts of protein deficient. The protein that results from combining two sources of incomplete proteins may be of higher quality. For vegetarians, incomplete protein is preferable to complete protein. as in Khichdi and Kheer.
The only incomplete protein in animal products is gelatin. In addition to having little leucine, it is deficient in three essential amino acids, including tryptophan, valine, and isoleucine. - Partially complete protein
Regarding the distribution of amino acids, partially complete proteins fall between full and incomplete proteins.
While they may not include all essential amino acids in sufficient quantities, they are protein sources that offer a more balanced profile of essential amino acids than normal incomplete proteins. Soy and quinoa are two typical examples of its origins.
Animal Sources: Meat, poultry, egg, dairy products like milk, curd & cheese
Plant Sources: Legumes, Nuts & seeds like almonds, peanuts, chia seeds, and flaxseeds, Soy based products like Tofu & tempeh, whole grains like quinoa, bulgur, and farro
On the basis of nutritional requirements, there are altogether 3 protein namely;
a. Complete protein
b. Incomplete protein
c Partially complete protein
Altogether there are 21 amino acids. Broadly they can be categorized as essential amino acids and non-essential amino acids. Out of 21 amino acids, there are 10 essential amino acids and 11 non-essential amino acids.
Altogether, there are ten essential amino acids: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine and Arginine (Additional for child)
